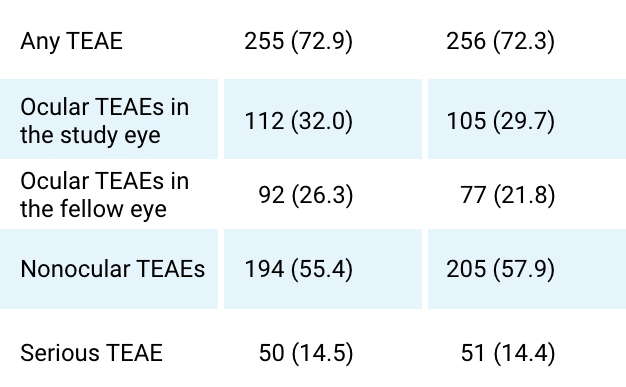
Summary of All Adverse Events up to Week 52
(Safety Set)2*
AE Summary
BYOOVIZ (n=350) n (%)
Lucentis (n=354) n (%)
TEAEs
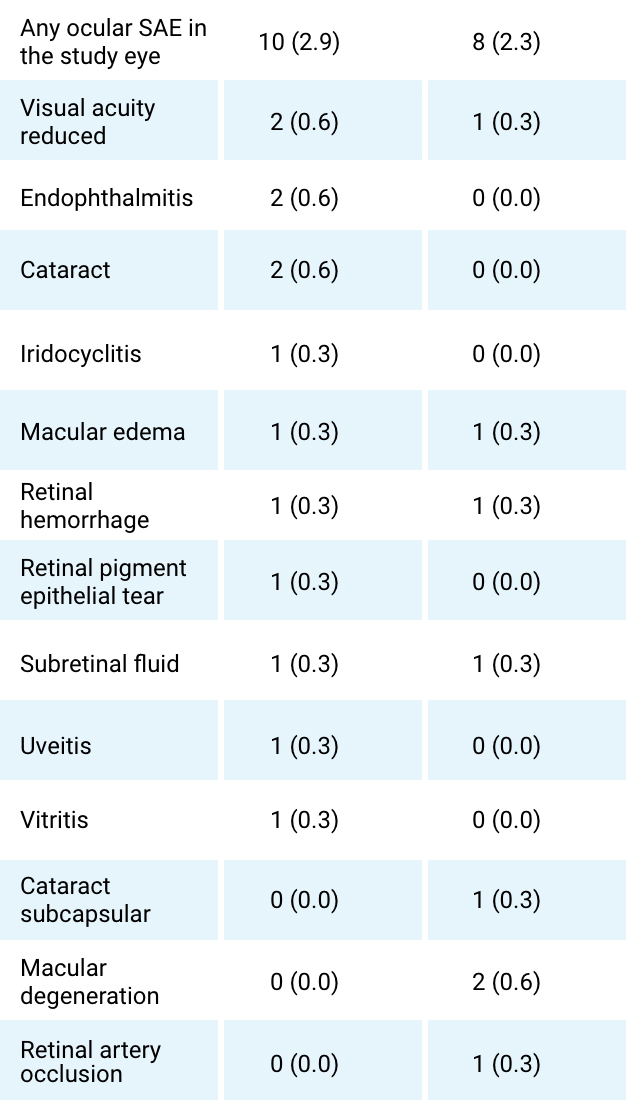
Serious ocular AE in the study eye (by preferred item)
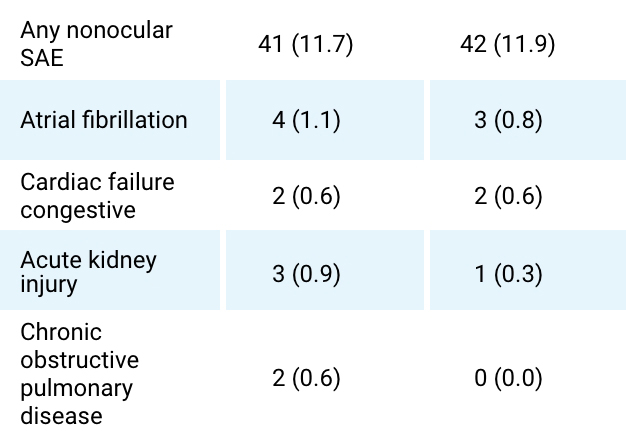
Serious nonocular AE (occurrence ≥0.5% in either treatment group; by preferred item)
AESI†

Any TEAEs leading to IP discontinuation
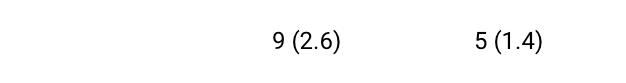
Deaths


- Adapted from Bressler NM, et al., 2021.
- Percentages are based on the number of participants in the safety set.2
- *
- Included participants receiving at least one dose during the study period after randomization.2
- †
- No definitive differences in the overall incidence of AESIs between treatment groups were identified. ‘Intraocular pressure increase’ occurred in 3 (0.9%) and 6 (1.7%) participants, while death was reported for 1 (0.3%) and 2 (0.6%) participants within the SB11 and RBZ groups, respectively. Furthermore, 2 (0.6%) and 4 (1.1%) participants in the SB11 group and none in the RBZ group experienced ‘Endophthalmitis’ or ‘Eye disorders’ which included iridocyclitis (n=3; 0.9%), uveitis (n=1; 0.3%) or vitritis (n=1; 0.3%).2
Additional safety, immunogenicity, and pharmacokinetic‡ (PK) outcomes were comparable between treatment groups1 up to Week 52 of treatment2
- ‡
- PK data were obtained in 54 patients and have limitations in their interpretation. Absorption of intravitreal injection anti-VEGF product into systemic circulation is very limited.2
- AE=adverse event; AESI=adverse event of special interest; SAE=serious adverse event; TEAE=treatment-emergent adverse event; VEGF=vascular endothelial growth factor.