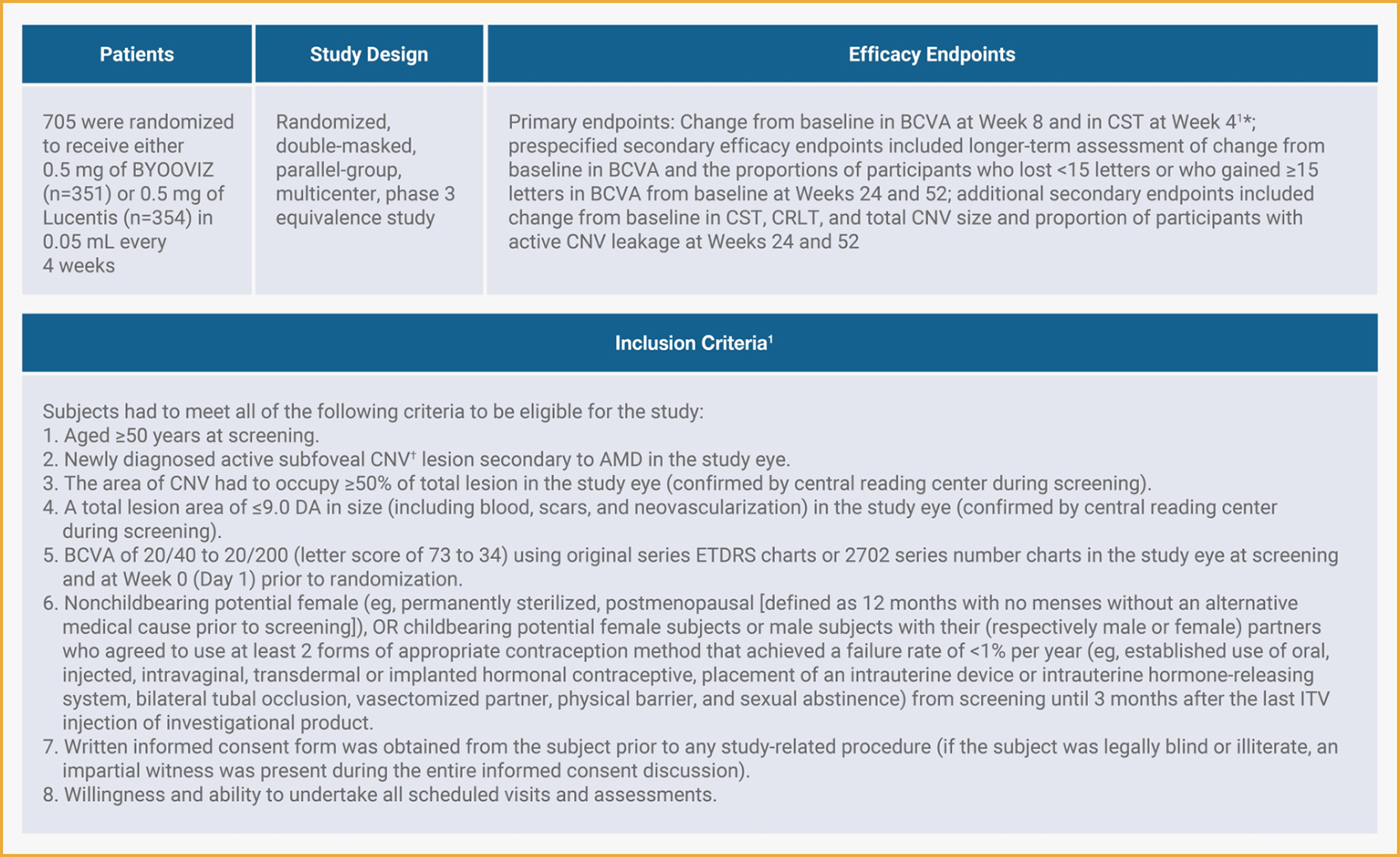
A Phase 3 Clinical Trial Comparing BYOOVIZ and Lucentis1,2
- *
- The endpoints were chosen in consultation with appropriate regulatory bodies. BCVA was the selected primary endpoint for FDA approval.1
- †
- Active CNV indicated presence of leakage and intra- or subretinal fluid and was confirmed by central reading center during screening.1
Primary Endpoint: Difference of LS Mean Change in BCVA Between BYOOVIZ and Lucentis at Week 81
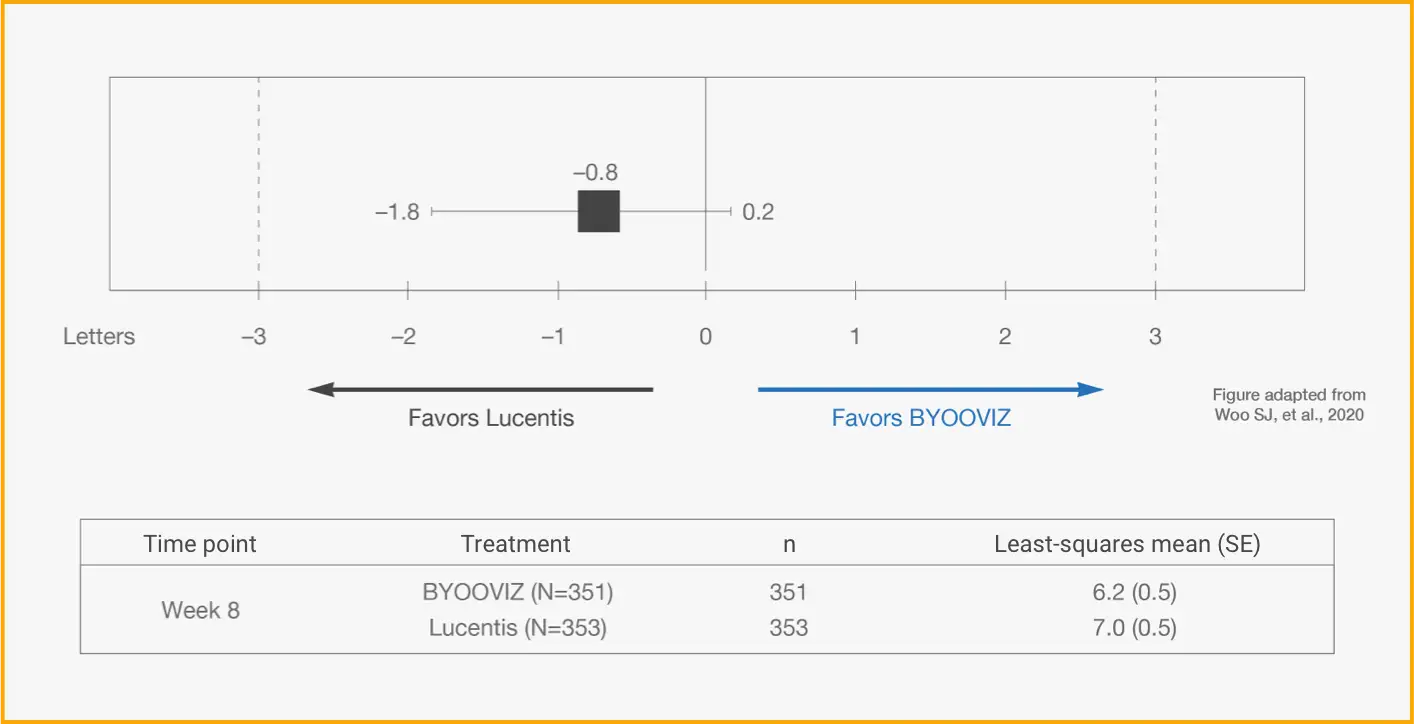
Difference in LS mean change from baseline in BCVA at Week 8 in the full analysis set (FAS) (BYOOVIZ − Lucentis); whiskers represent the 90% CI that is contained within the predefined equivalence margins of −3 to 3 letters, represented by the dashed lines.
n=351 (BYOOVIZ), n=353 (Lucentis). The FAS included all randomized participants, excluding 1 inadvertently randomized participant who did not receive study drug.1
- At Week 8, visual acuity for BYOOVIZ was within the predefined equivalence margins compared with Lucentis1
- Adjusted treatment difference between groups was −0.8 letters (90% CI: −1.8 to 0.2 letters)1
Primary Endpoint: Difference of LS Mean Change in CST Between BYOOVIZ and Lucentis at Week 41
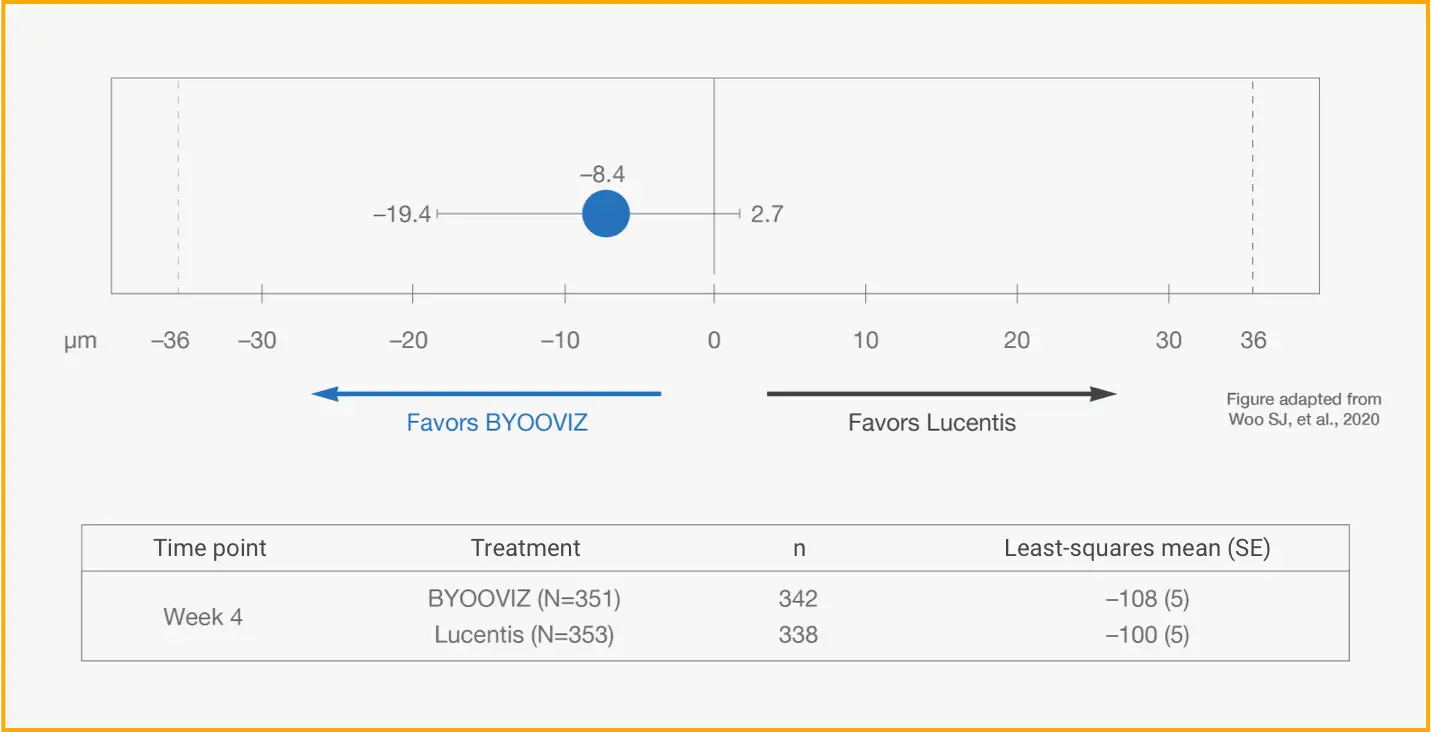
Difference in LS mean change from baseline in CST at Week 4 in per-protocol set (PPS) (BYOOVIZ – Lucentis); whiskers represent the 95% CI that is contained within the predefined equivalence margin of −36 to 36 μm, represented by the dashed lines.
n=342 (BYOOVIZ), n=338 (Lucentis).
- At Week 4, CST for BYOOVIZ was within the predefined equivalence margins compared with Lucentis1
- Adjusted treatment difference between groups was –8.4 μm (95% CI: –19.4 to 2.7)1
1-Year Study: Change From Baseline in BCVA at Each Time Point Through Week 52 in the FAS2*
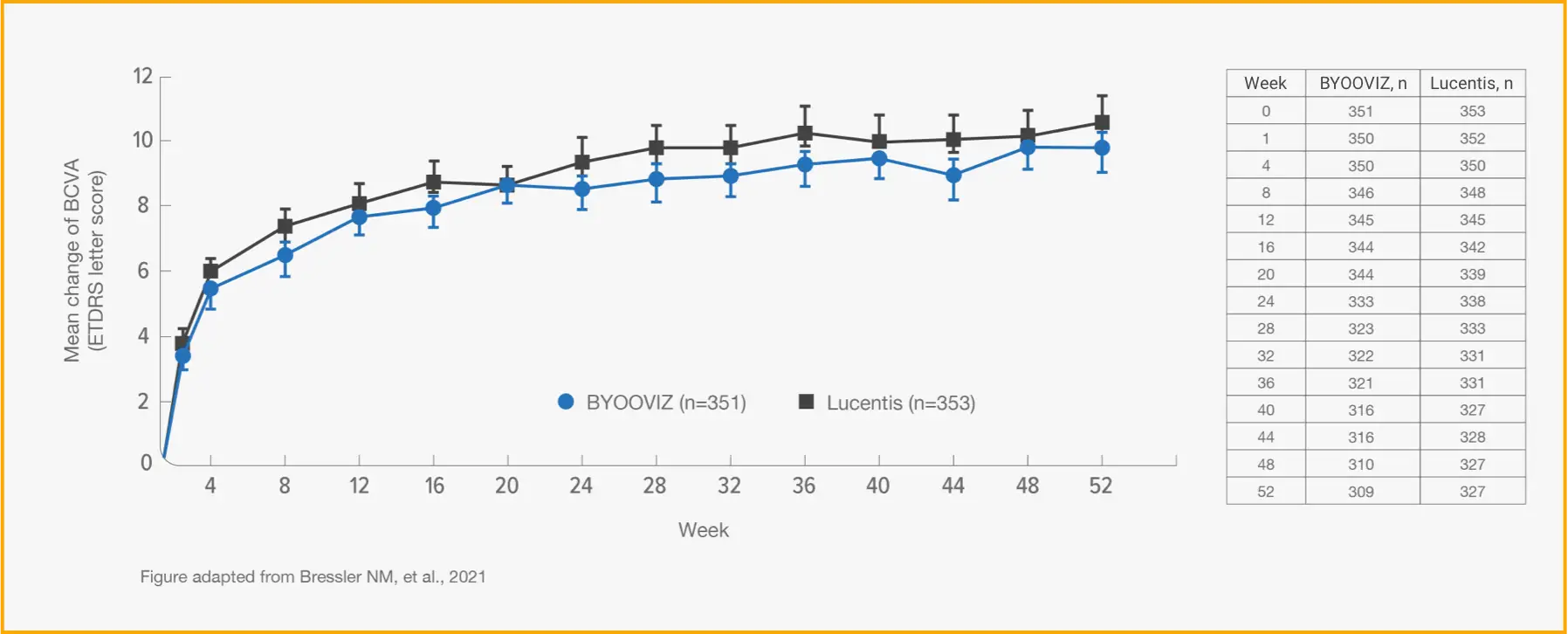
TAP TO EXPAND TIME POINTS THROUGH WEEK 52

Mean (SD) change from baseline in BCVA for participants who completed Week 52 of the study: BYOOVIZ (n=309), 9.8 (11.4) letters; Lucentis (n=327), 10.4 (11.5) letters. Circles and squares represent mean and error bars represent SE at each time point.
- *
- For additional information and all other secondary endpoints, please refer to Bressler NM, et al., 2021.2
- Change from baseline in BCVA was 9.8 letters for BYOOVIZ and 10.4 letters for Lucentis at Week 52 (adjusted treatment difference [SE]: −0.6 [0.9]; 90% CI: −2.1 to –0.9 letters)2
- The proportion of participants who lost <15 letters and who gained >15 letters was comparable between treatment groups at all time points2
1-Year Study: Change From Baseline in CST at Each Time Point Through Week 52 in the FAS2
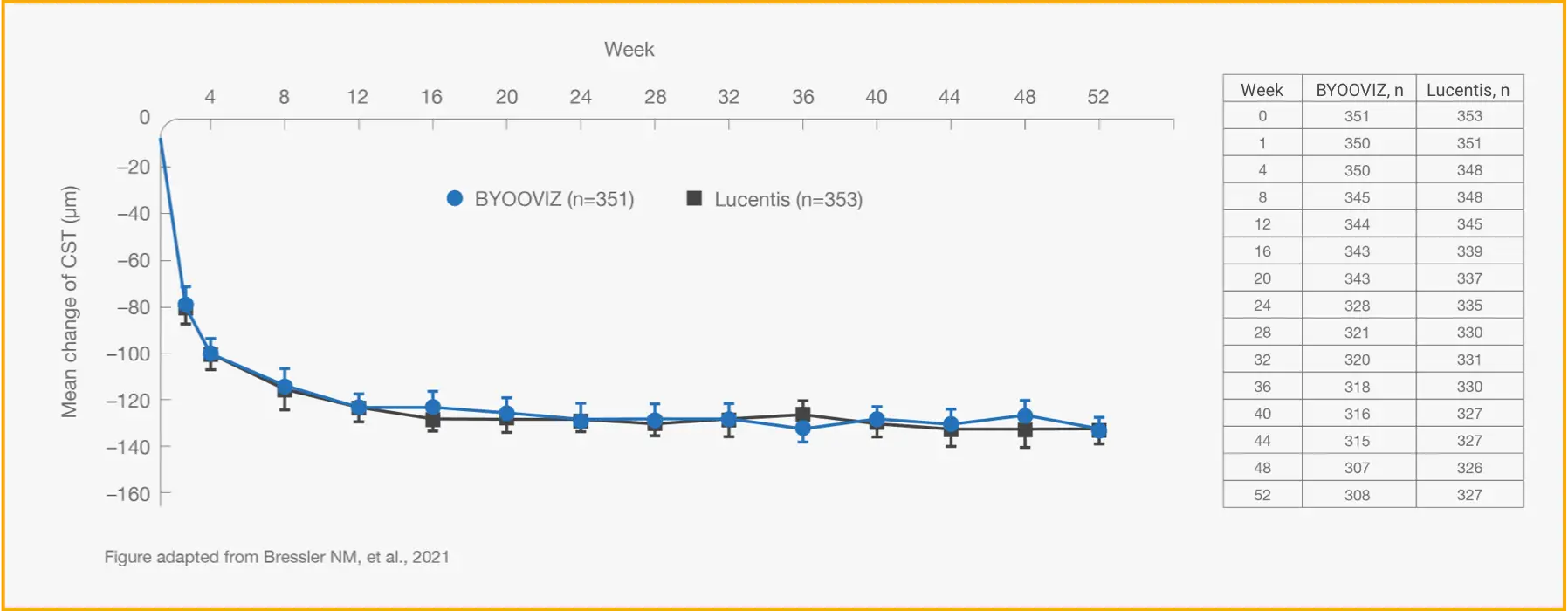
TAP TO EXPAND TIME POINTS THROUGH WEEK 52

Mean (SD) change from baseline in CST for participants who completed Week 52
of the study: BYOOVIZ (n=308), –133.6 (103.9) μm; Lucentis (n=327), –128.4
(116.1 μm). Circles and squares represent mean and error bars
represent SE at each time point.
- Change from baseline in CST was −140.0 μm for BYOOVIZ and −125.1 μm for Lucentis at Week 52 (adjusted treatment difference [SE]: −14.9 [5.3]; 95% CI: –25.3 to –4.5)2
- The change from baseline in CST was comparable between treatment groups at all time points up to Week 522
BCVA=best corrected visual acuity (ETDRS letter score); CI=confidence interval; CNV=choroidal neovascularization; CRLT=central retinal lesion thickness; CST=central subfield thickness; DA=disc area; ETDRS=Early Treatment Diabetic Retinopathy Study; ITV=intravitreal; LS=least squares; nAMD=neovascular (wet) age-related macular degeneration; SD=standard deviation; SE=standard error.